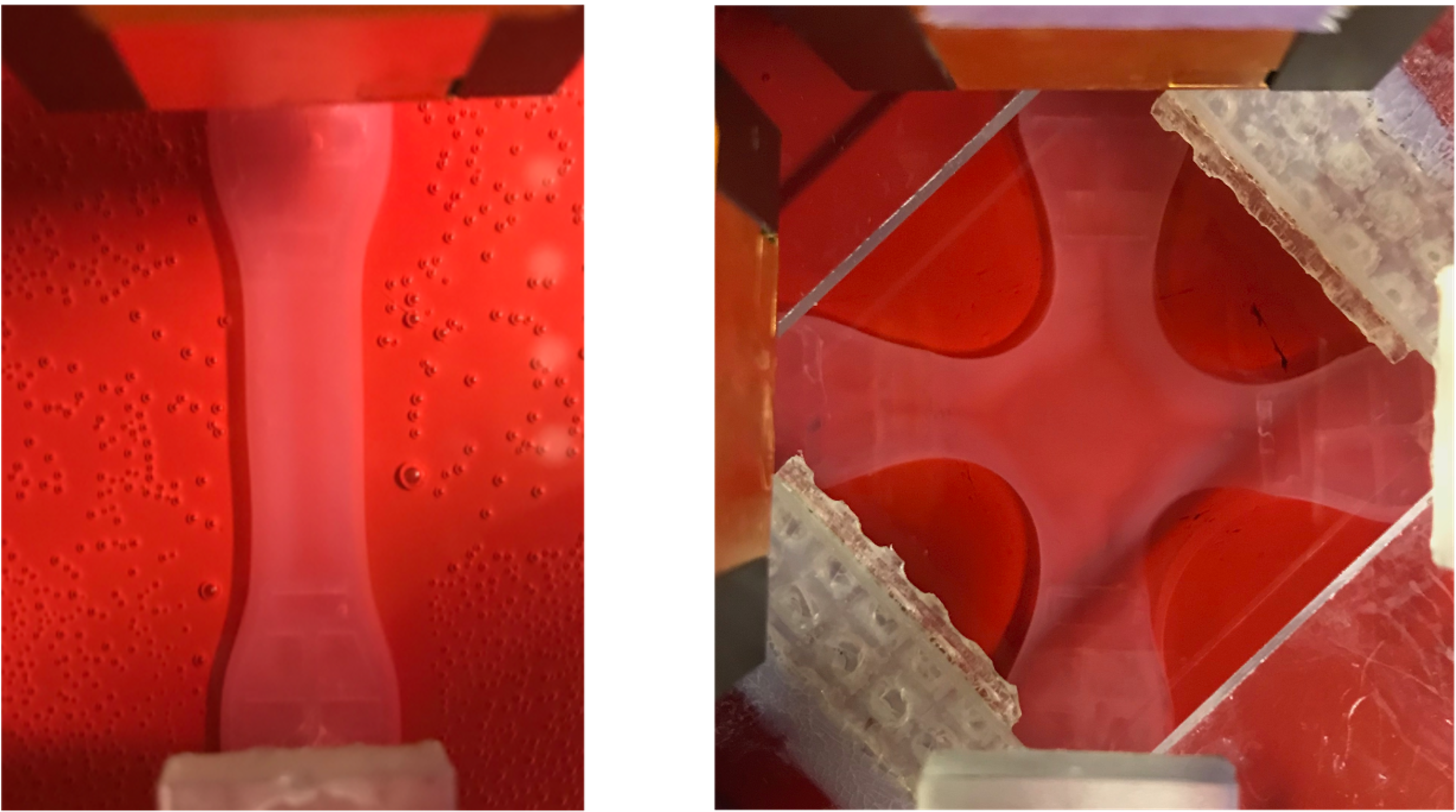
Motivation
Soft biological tissues consist of cells and an extracellular matrix (ECM) – a fibrous network of different proteins. The cells can actively sense their surrounding ECM and regulate its mechanical state to maintain a preferred – also called homeostatic – state. Researchers use cell-seeded collagen gels to study these interactions, but there is limited quantitative data on the stresses or forces cells generate in these gels, which are mostly derived from uniaxial experiments despite tissues experiencing multiaxial loading in the body.
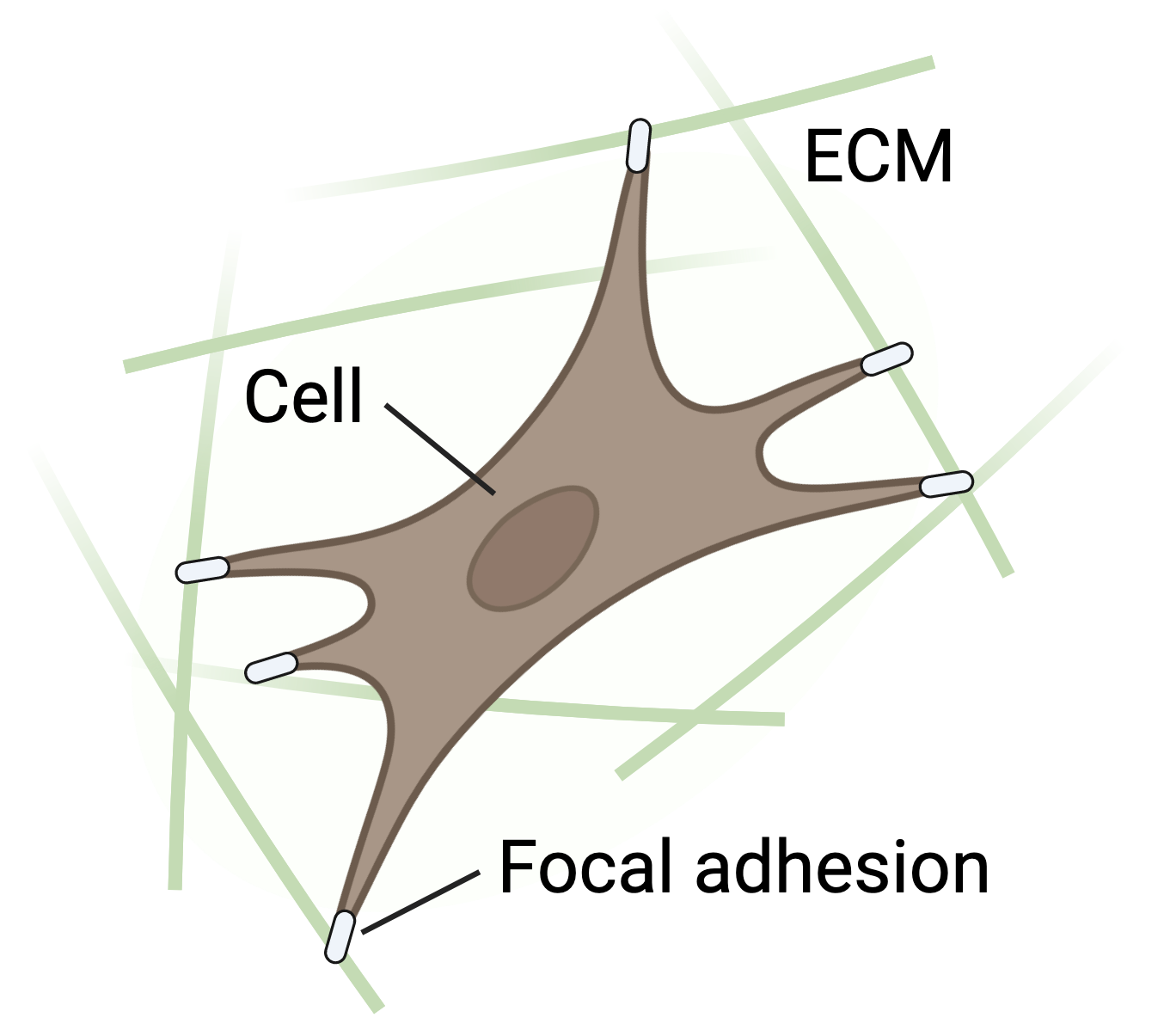
Methods
To bridge the gap between current data and in-vivo conditions, we developed a computer-controlled bioreactor that reliably measures cell-generated forces in tissue equivalents under biaxial loads. Typical forces are in the range of 0.1 to 1 millinewtons (mN) – approximately equivalent to the weight of a postage stamp. This device enables studies on how cells establish and maintain a preferred mechanical state and how various factors, like the number of cells, growth factors, and different loading conditions, affect these processes.
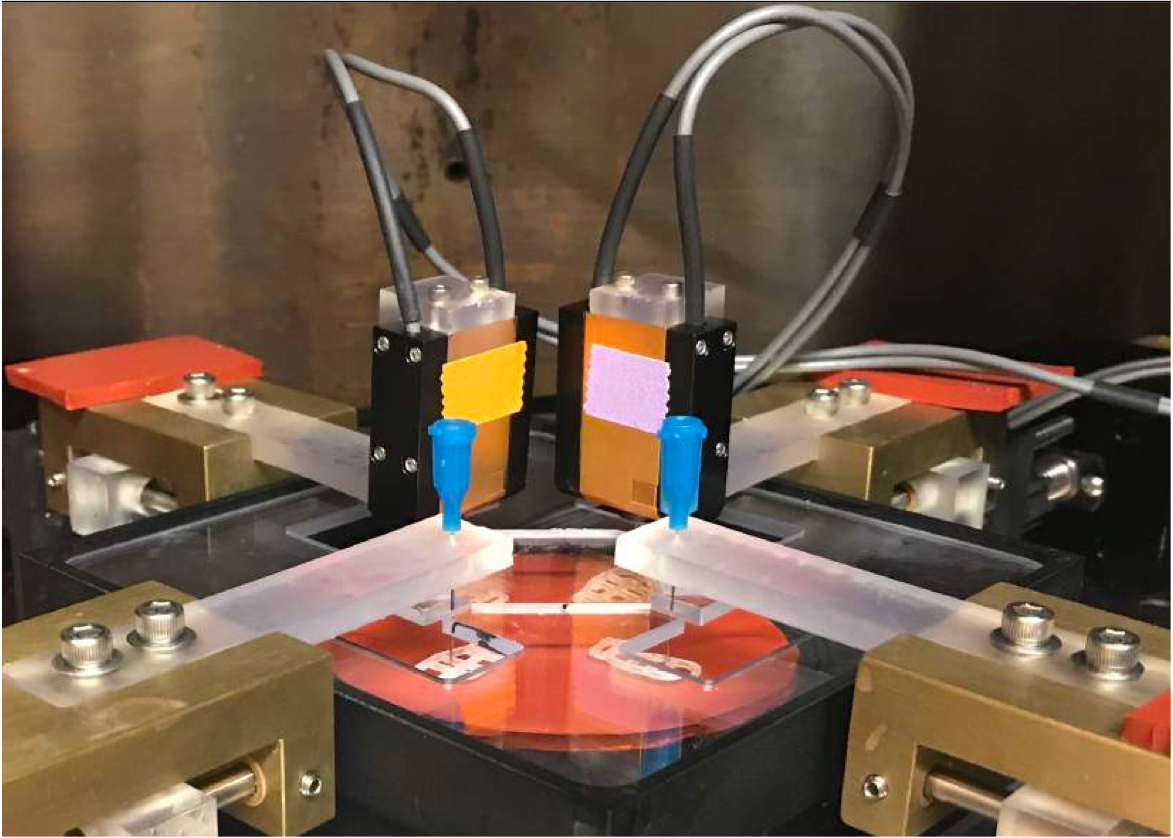
Results
To test and validate the device, we show that fibroblasts – a common cell type in connective tissue – establish a homeostatic mechanical state that depends on cell density and collagen concentration. Following perturbations from this homeostatic state, the cells were able to restore a mechanical state similar to the one prior to the perturbation. Depending on the applied loads, however, they were not always able to fully maintain that state.